Data, they say, is the new gold. All sectors of the economy continue to collect huge amounts of data to serve humanity better. And the healthcare sector, among other sectors, arguably tops the list regarding data collection.
Many data collected in the healthcare sector come from various sources, including radiology information, insurance portals, lab information systems, ERPs, and clinical trial applications. With so much data to be processed, integrating this information across all channels in the health sector has become labor-intensive and complicated. This ultimately has increased the consumption of resources, hence, reducing efficiency.
To tackle these challenges, many healthcare companies are now bringing Robotic Process Automation (RPA) into the picture to automate repetitive and redundant procedures vital to their system’s smooth functioning.
One of those processes is Clinical Trials. In this article, we look at ways in which RPA can help provide credible data and reduce cost, workforce utility, enhance operational efficiency, and restrict the likelihood of errors.
Learn more about using Robotic Process Automation in other industries, like RPA in insurance or RPA in logistics.
Clinical trials – how the processes look like?
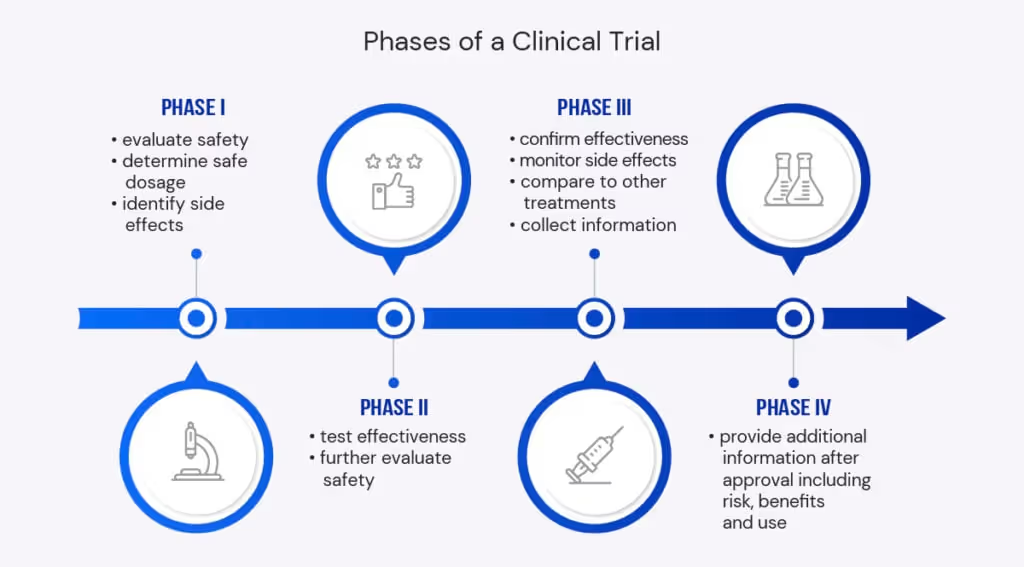
Clinical trials are research studies that aim to deduce if a treatment, device, or medical strategy is safe for human consumption and use. In general, clinical studies provide credible data and medical knowledge, which assist in making healthcare guidelines and decisions.
The main objective of clinical trials is research. Trials are modeled to add to medical data and knowledge pertaining to diagnosing, preventing, and treating disease conditions.
When it comes to healthcare research, a successful clinical trial needs a lot of accurate and precise data computations. No study or research is better than the quality of data used. The quality of data used in the stages of clinical trials is the most crucial factor to its success. To this end, many healthcare companies continue looking for ways to achieve and use high-quality data in every clinical trial stage.
There’s been a continuous increase in clinical data management efforts, which are reflected in related healthcare guidelines, books, and publications. However, as much as new measures are being made, there are still several sources of errors in clinical trials regarding data entry, collection, and processing.
How automation and RPA help to improve the efficiency of clinical trials
Robotic Process Automation (RPA) continues to be an innovative and effective technology across many industries within the economy, automating repetitive and manual processes. Given that RPA focuses on integrating several IT systems, improving employee productivity, and eliminating human error, RPA has become a useful technology in the Life Sciences industry, especially in clinical trials.
As explained earlier, Randomized Clinical Trials (RCT) are the standards for evaluating new therapeutics in terms of safety when used in human subjects. Presently at a success rate between 40% to 80% across various phases, clinical trials are still considered to have significant failures due to patient recruitment.
Furthermore, with many challenges to overcome when conducting RCTs, the cost of trials is still very high. Some challenges that affect RCT include study design, trial sites, patient retention, availability of the patient, and principal investigators. However, with the integration of RPA, these challenges can be checked, thereby increasing the efficiency and success rate of clinical trials.
Robotic Process Automation can improve rule-based processes and workflows in clinical trials. This is because the bots can manipulate data, transaction processes, and system calibration facilities while improving storage.
The benefits of RPA to clinical trials
Eliminate Monotonous Manual Data Collection
Robotic Process Automation (RPA) brings about infinite possibilities. Through the integration of RPA, Life Sciences companies can effectively speed up the duration of the vaccine market while enhancing regulatory measures and safety.
Through the integration of RP, valuable time is saved during clinical trials, freeing healthcare professionals to perform other tasks. RPA effectively promotes efficiency by providing a continuous, error-restructured data collection technique.
Automate Analysis To Show Extensive Effect Overview
With RPA, the Life Science industry can adequately integrate quick data to analyze and detect the overall effect of formulated drugs and vaccines. The patients will be able to notify RPA bots of all side effects. These robots, in turn, then combine this data with medical historical data and external sources such as location-based data or weather to provide a general overview for additional analysis.
After introducing such a vaccine or drug into the market, the same RPA bots also assist in analyzing the side effects of such drugs that were not predetermined. These side effects, including market sentiments, are collected daily and integrated with previous analyses. This is to continuously manage and monitor the vaccine’s impact on the market.
At the end of it all, integrating RPA bots enhances operational efficiency and improves the management of clinical trial audit logs and compliance with pharmacovigilance regulations.
Assist In Validation Processes
RPA bots can be integrated to be involved in validation processes. However, the process of integration is not that simple. To program bots to validate various clinical methods, stringent regulations are required for each separate system that supports the development of medicine or vaccines.
Therefore, a well-structured robot configuration framework, change management, and validation are required to prevent confirmation errors. This is very crucial to the process of integrating RPA solutions.
Other benefits include:
- Facilitates quick response to patience, which helps to improve the progress of both the doctor and the patient.
- Provides patients with one-point access to their data profile
- Assist in scheduling appointments and make immediate reminders for the patient and the doctor.
- Facilitates healthcare professional cross-platform access to information
RPA Clinical Trials Use-cases
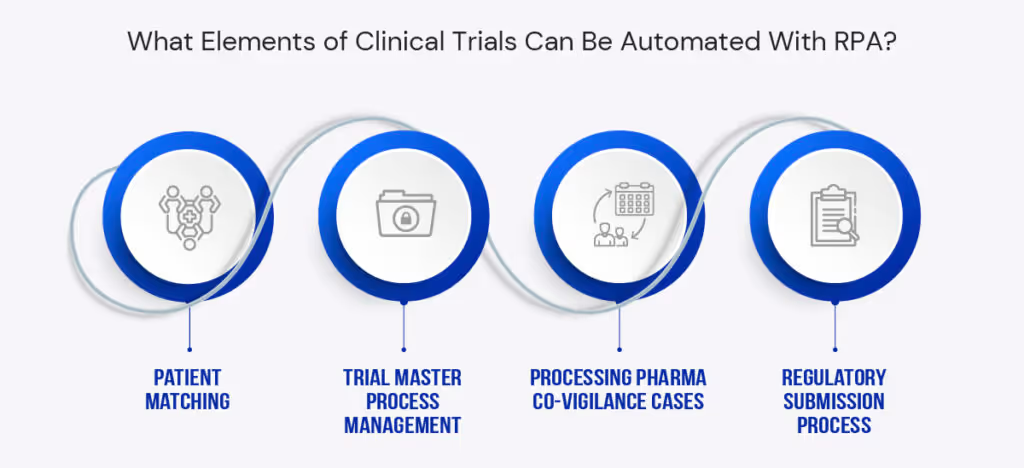
Business endpoints that can be optimized using RPA are:
Patient Matching
Creating a patient population using dynamic exclusion and inclusion to evaluate the effect of a trial is usually overwhelming. However, the integration and use of RPA effectively perform this task. As a result, the eligible patient population can be easily enlarged by the study designer if there’s a need for modification.
Automating patient matching with RPA makes the entire process better and faster. RPA effortlessly increases recruitment speed by carrying out initial execution with potential patients before the decisive interaction with associates of the clinic. RPA assists in lessening the effort put into repetitive tasks at the patient recruitment stage for a clinical study.
Processing Pharma Co-vigilance Cases
Based on a study by ClinicalResearchNewsOnline.com, it is estimated that big pharmaceutical companies, on average, process about 700,000 adverse incident cases annually. With this kind of pressure on big Pharma companies, a lot of these companies plan to go lean to reduce costs while increasing the caseload and not jeopardizing the base cost.
Today, about 50% of the PV resources are utilized to manage cases needing data integration. However, according to RoboticsandAutomationNews.com, automation of these manual steps can reduce the time spent on PV by 45%, saving big Parma companies a lot of money yearly.
Trial Master Process Management
Sponsors of clinical trials are known to record every activity carried out in a clinical study. This includes various sites in a master data repository called Trial Master File (TMF).
Presently, these data are still manually inputted into the TMF structures. As a result, sponsors with many CROs have to create multiple TMFs, which are often not efficiently integrated. Errors during this integration are known to limit the level of insights drawn from the TMF. That’s not all; additional resources must be trained and equipped to verify and maintain each system.
However, with more healthcare companies integrating RPA, the trial controller process management is carried out quickly and efficiently. This is because uploading data and documents into the TMF becomes automatic. The integration of RPA has proven to lessen the time spent on data entry by a whopping 90%, saving millions of dollars on each clinical trial every year.
Regulatory Submission Process
The process of submission for regulatory activities is usually slow. It involves pharmaceutical companies participating in activities like compiling health records and monitoring the status of documents. Automating this process via RPA has been observed to reduce the time to market a product significantly.
Conclusion
It has become obvious that RPA is the answer to increasing the efficiency of repetitive but vital administrative tasks in clinical processes. RPA is gradually enhancing the efficiency of pharmaceutical firms in focusing on producing effective and safe drugs at a meager cost to the market.
Learn more about the processes in the healthcare industry that can be automated.
RPA now effortlessly assists in preventing delays in the introduction of drugs into the market. It also helps to redistribute tasks of higher values, such as core research and development, to employees. At Flobotics, we give a wide range of RPA consulting services; help recognize automation opportunities, analyze these healthcare processes, establish essential infrastructures, and develop sustainable and stable bots.











.jpg)


